Limbic Encephalitis and Discovery of Novel Autoantibodies
In the last decade, autoantibody-associated autoimmune encephalitis has been increasingly recognized in patients with acute symptomatic seizures and epilepsy. The target antigens can be either surface or intracellular, which is of clinical relevance because the pathogenesis and prognosis are variable. However, specific autoantibodies can only be detected in a subset of patients clinically suspected of having autoimmune encephalitis (AIE). Some patients remain seronegative for known autoantibodies, but as patients with AIE particularly benefit from a specific tailored immunotherapy, the identification of new autoantibodies is crucial for optimal patient stratification.
Our goal is therefore to discover novel autoantibodies through improved screening of blood serum. Our screening for novel autoantibodies has already identified the novel antibody target Drebrin, which is a neuronal scaffold protein with a strong impact on neuronal function (Pitsch et al., 2020).
We have established an experimental identification paradigm for previously undetected autoantibodies in sera from patients who are seronegative for previously known autoantibodies but clinically suspected of having AIE. We use a wide range of methods, mainly in vitro techniques, including immunoprecipitation, western blotting, cloning, protein expression and purification, neuronal cell culture, immunohistochemistry, confocal microscopy, mass spectrometry etc.
Our overall goal is to identify and characterize additional potentially disease-relevant autoantibodies associated with AIE. Based on the knowledge of new autoantibody targets, we will derive new pathogenetic concepts for AIE for further investigation in the future.
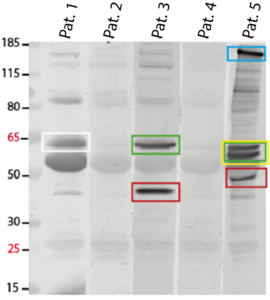
Figure: Identification of novel autoantibodies in clinically suspected AIE patients. Immunoblot of brain homogenates incubated with serum from three representative patients (#Pat.1, 3, 5) revealing strong bands at different sizes (colored rectangles) resembling unknown antigens as they are not visible in negative control samples (#Pat.2, 4). Those positive bands will be further processed and proteins will be identified by mass spectrometry.
Identifying the Role of T Cells in Limbic Encephalitis
Limbic encephalitis (LE) encompasses a spectrum of inflammatory changes in affected brain structures, including the presence of autoantibodies and lymphoid cells. However, the mechanisms that trigger the major clinical pathological sequelae of LE, which may induce temporal lobe epilepsy (TLE) with chronic spontaneous seizures and hippocampal sclerosis, remain unresolved. The development of in vivo models that mimic the progressive hippocampal damage leading to hyperexcitability could greatly contribute to the understanding of key effectors in the disease. Recently, we established a model for the expression of immunogenic proteins in the mouse hippocampus using molecular biology approaches (Pitsch et al., 2021). This model will allow us to study the role of cytotoxic T cells and identify the key genes and effector cells involved in the immune response and subsequent neuronal hyperexcitability. Therapeutic strategies to attenuate the expression of the disease can then be evaluated.
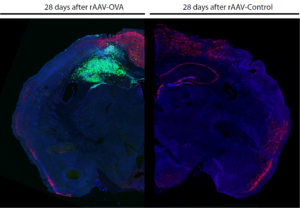
Figure: Consequences of limbic encephalitis induction after 28 days. Comparison between limbic encephalitis induced hippocampus (left), with local accumulation of T cells (green) and neuronal cell loss (neurons = red), and a healthy hippocampus (right) without any T cells.
Pitsch, J., van Loo, K.M.J., Gallus, M., Dik, A., Kamalizade, D., Baumgart, A.K., Gnatkovsky, V., Muller, J.A., Opitz, T., Hicking, G., et al. (2021). CD8(+) T-Lymphocyte-Driven Limbic Encephalitis Results in Temporal Lobe Epilepsy. Ann Neurol 89, 666-685.
Characterization of Novel Autoantibodies
Autoimmune encephalitis (AIE) is increasingly recognized as a trigger of acute symptomatic seizures or epilepsy. In many patients, AIE is caused by specific autoantibodies directed against various neuronal structures such as receptors, enzymes and parts of the cytoskeleton. One of these new antibodies related to AIE is anti-drebrin, which we have already started to investigate (Pitsch et al., 2020). This autoantibody is directed against the body’s own intracellular scaffolding protein Drebrin (Developmentally REgulated BRain proteIN), which is located on neuronal dendritic spines. For the first time, a functional significance of an intracellular binding autoantibody could be demonstrated here, which leads to an impairment of synaptic structures as well as to a hyperexcitability of the neuronal network.
We are aiming to understand in detail the pathological consequences of LE-related antibodies targeting different key molecules in neuronal functionality and structure. Therefore, we are using cultured primary hippocampal neurons and murine organotypic hippocampal slice cultures (OHSCs).
On cellular and network levels, we are investigating the effect of LE-related autoantibodies on different aspects of neuronal structure such as synaptic distribution, neuronal connectivity and dendritic arborization. Furthermore, we are testing for antibody uptake by using different internalization assays such as varying temperatures or epileptiform solution. In order to investigate different effects of LE-related autoantibodies on neuronal functionality, we examine neuronal network activity in vitro, differing release of neurotransmitters and potential uptake of neuronal receptors.
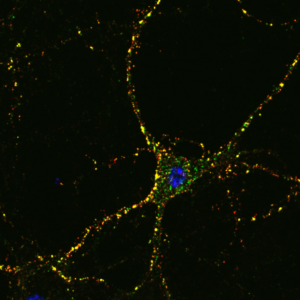
Figure: Incubated cultured primary hippocampal neuronal neuron. Internalized surface receptors (green) and surface receptors (neurons = red) after an internalization assay.
Pitsch, J, Kamalizade, D, Braun, A, Kuehn, JC, et al. (2020). Drebrin Autoantibodies in Patients with Seizures and Suspected Encephalitis. Ann Neurol 87, 869-884.