Neurodevelopmental epileptogenic lesions
- Focal cortical malformations or epilepsy associated tumors, such as gangliogliomas.
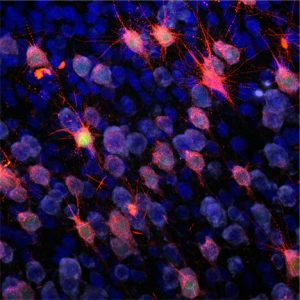
- Focal Cortical Dysplasias characterized by defects in cortial organization and architecture, aberrant proliferation and differentiation as well as intractable epilepsy and mental retardation.
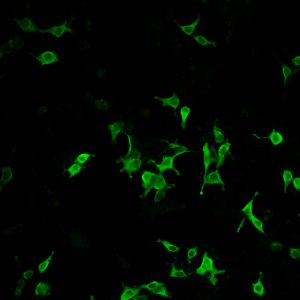
- Gangliogliomas are the most common epilepsy associated tumors in pharmacoresistant patients with focal epilepsies, composed of dysplastic neurons and proliferative, neoplastic glial cells.
- Use of in-vivo models and human organotypic slice cultures to unravel basic principles of ontology and epileptogenesis as well as development of new treatment concepts.
Acquired insult induced epilepsies including limbic encephalitis
- In many patients with focal epilepsies, the cause of epilepsy is still enigmatic.
- Limbic encephalitis (LE) has been diagnosed in a significantly increased number of mainly adult patients.
- LE present frequently with sudden onset of temporal lobe epilepsy accompanied by a serious impairment of their episodic memory.
- The syndromes are characterized by auto-antibodies against neuronal antigens, onconeural auto-antibodies and anti-GAD65.
- Analyze the spectrum of auto-antibodies in distinct LE-patient cohorts.
- Research interests in novel auto-antibodies in LE.
- Study role of distinct inflammatory components including cytotoxic T-cells.
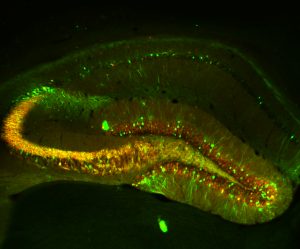
Synapto- and Channelopathies in epileptogenesis and innovative therapeutic strategies
- Transcriptional channelopathy of T-type Ca2+ channel subunit CaV3.2
- Identify the molecular signaling cascades involved in CaV3.2 transcriptional regulation.
- Study the role of zinc for of T-type Ca2+ channelopathies in epilepsy.
- Understand the role of distinct transcription factors including EGRs, MTFs orchestrating Ca2+ channelopathies in epilepsy
- Develop new strategies for pharmacological intervention in TLE pathogenesis, including the design of gene/pathway-specific drugs.
- Apply CRISPRa/i as well as ASOs targeting distinct epileptologenesis-relevant molecules in in-vivo models and human organotypic slice cultures.